By Nina Bai
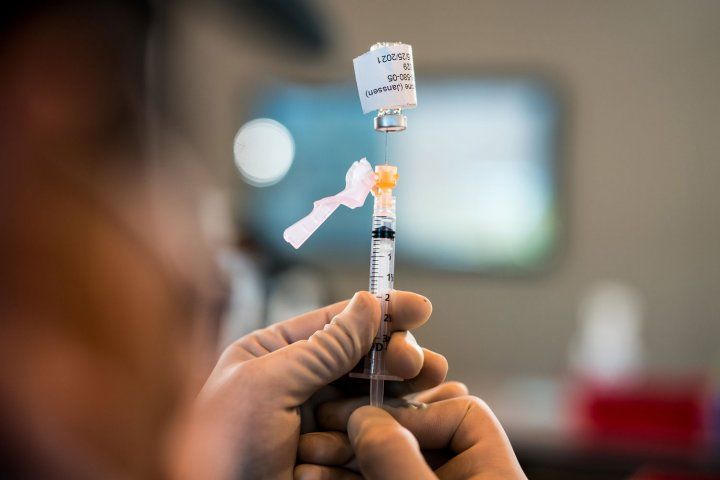
The Johnson & Johnson one-dose vaccine received FDA authorization on Feb. 27. Photo by Maurice Ramirez
Editor's Note: This story was updated May 7 to include new information on the risk of blood clots.
With the FDA’s emergency use authorization of the Johnson & Johnson vaccine on Feb. 27, the U.S. now has three highly effective vaccines against COVID-19.
All three vaccines – Johnson & Johnson, Pfizer, and Moderna – completely prevented hospitalizations and deaths in clinical trials, which is why public health experts are recommending that people get the first vaccine that is available to them. But the vaccines differ in a number of details of how they work and how they were studied, leading to questions and some confusion.
We asked UC San Francisco infectious disease expert Monica Gandhi, MD, MPH, to unpack some of the big questions around vaccine science, such as how the Johnson & Johnson vaccine differs, how well it works against the new variants, and whether you should be worried about transmitting the virus after vaccination.
Use of the Johnson & Johnson (J&J) vaccine has resumed after a pause recommended by the FDA and CDC in order to study reports of severe blood clots. What are the risks of this rare side effect?
As of May 6, 2021, 16 cases of these blood clots have been reported among a total of about 8 million people who received the J&J vaccine. The unusual condition is known as thrombosis (blood clot) with thrombocytopenia (low platelet count), or TTS, which normally occurs at a rate of about one in a million per year in the general population. Among those who have received the J&J vaccine, the rate is about two in a million. That is on par with the rate of anaphylaxis, a severe allergic reaction, that has been reported for the mRNA vaccines, about 2.5 per million.
The risk appears to be higher in younger women: 15 of the confirmed cases have been in women, and 13 of these in women ages 18 to 49. Among women ages 18 to 49, the risk of TTS after the J&J vaccine is about seven in a million.
“So that does put the risk in context, in the sense that it is still extremely low,” said Gandhi.
TTS also has been reported after the AstraZeneca vaccine in Europe, but it’s not yet clear in either case how the vaccines may be causing these blood clots. Similarities with an autoimmune condition known as heparin-induced thrombocytopenia (HIT) suggests the body is producing antibodies against its own platelets.
Are there people who should avoid taking the J&J vaccine?
The FDA has added a warning about TTS in its emergency use authorization of the J&J vaccine but has not restricted its use. “I think it’s prudent to put a warning label on it,” said Gandhi. “The warning label helps patients to speak with their doctor about the blood clot risk and also helps physicians to be alert to such occurrences.”
The reported cases of TTS have occurred one to two weeks after the J&J vaccine and most often have involved blood clots in the veins of the brain, but also elsewhere in the body. The symptoms to watch out for include severe headache, back pain, abdominal pain, blurry vision and other neurological symptoms. The condition can be monitored and treated, said Gandhi.
Gandhi said she has not advised her own patients against taking the J&J vaccine, except a few younger women who are on oral contraceptives, which already increases the risk for blood clots. For those patients, she recommends the mRNA vaccines.
Overall, Gandhi said the pause has not diminished her enthusiasm for the J&J vaccine. “It’s a very effective vaccine,” she said, “And I think it’s an important vaccine for the world, especially when we see what’s going on in India right now.”
The fact remains that continued use of the J&J vaccine will reduce hospitalizations and deaths. “I keep coming back to how very rare TTS is and how very common COVID is,” said Gandhi. “You put together your risk versus benefit analysis and it’s much more common to be exposed to COVID than it is to this very rare side effect.”
How does the J&J vaccine work? How is the biological mechanism different from that of the mRNA vaccines?
All three vaccines work by prompting your own cells to produce the spike protein – the protrusions on the coronavirus that allow it attack human cells – so your immune system learns to recognize and respond quickly to a real infection.
Unlike the mRNA vaccines from Pfizer and Moderna, which deliver fat-covered bits of genetic material into your cells, the Johnson & Johnson vaccine uses a “shell of a virus” to carry genetic material into your cells, said Gandhi. The “shell” is an adenovirus, which normally causes colds, but has been modified so that it can no longer replicate and make you sick.
The genetic material in the mRNA vaccines is RNA, whereas the genetic material in the J&J vaccine is DNA, but both encode the information to make the spike protein of SARS-CoV-2.

Johnson & Johnson vaccine doses being prepared at the City College vaccination site in San Francisco. Photo by Maurice Ramirez
“Inside the adenovirus is the DNA that your body will use to make into RNA and then into the spike protein of the coronavirus,” said Gandhi. She noted that the DNA and RNA then quickly degrade in the human body and do not have the ability to affect our chromosomes.
Once your cells make the spike protein, all three vaccines work similarly. “The spike protein doesn’t look like anything in our human body,” said Gandhi. “So, you raise an immune response with T cells and antibodies to that spike protein, and that allows you to fight the virus if you ever see it in the future.”
This type of adenovirus technology has been used in an Ebola vaccine but is still relatively new. “It’s more familiar than mRNA technology, but I wouldn’t say it is the most familiar technology either,” said Gandhi.
Why is the J&J vaccine only one dose? Is it possible that it could eventually become two doses or that the mRNA vaccines could become one dose?
The J&J vaccine went into Phase III trials as a one-dose vaccine because earlier phase trials had shown strong immune responses after just one dose, said Gandhi. “After one dose, across all populations, even in older people, the antibody response and T-cell response were excellent and increased over time.”
J&J is currently conducting a trial to give people two doses, which may improve efficacy against mild and moderate disease, said Gandhi.
Gandhi also noted that the J&J vaccine continues to increase in efficacy after two weeks, the current CDC-recommended waiting period after vaccination. Immunogenicity data from the Phase I/II trials suggest the immune response may continue to improve even after 28 days. “I do think it’s going to have even more efficacy after four weeks,” said Gandhi. “I call it the gift that keeps on giving.”
For the mRNA vaccines, the immunogenicity data after one dose did not look as powerful, so the Phase III trials were conducted with two doses. Although some real-world data suggests that one dose may also offer good protection, Gandhi said there is not enough information to recommend getting only one dose of the mRNA vaccines.
Some people are concerned that the overall efficacy of the J&J vaccine – 66 percent globally, 72 percent in the U.S. – is lower than the 94-95 percent reported for the mRNA vaccines. Can you put this difference in context?
“The way I think about these vaccine trials is to look at the worst outcomes first – hospitalizations and deaths from COVID-19 – because that’s what got us into trouble to begin with,” said Gandhi.
In the J&J trial, the placebo group had 16 hospitalizations and seven deaths from COVID-19, whereas the vaccine group had none, which means the vaccine provided 100 percent efficacy against hospitalizations and deaths.
For severe disease, which includes people who were sick enough with COVID-19 to require medical intervention but recovered without hospitalization, the efficacy was about 85 percent across the board in Brazil, South Africa and the U.S.
Including mild and moderate disease, the overall efficacy was 66 percent, but varied across the regions: 72 percent in the U.S., 64 percent in South Africa, and 61 percent in Brazil. “Mild and moderate outcomes” could include a range of illness, said Gandhi, and we won’t know the details until the full trial results are published, but we do know that everyone recovered without medical intervention.
“Admittedly, against mild to moderate disease, it didn’t work as well, and I understand people’s concerns,” said Gandhi.

Cars lined up at the the City College drive-through vaccination site in San Francisco. Photo by Maurice Ramirez
Yet she said that on a population level, the availability of a one-dose vaccine can really speed up vaccinations and help bring total cases down. She pointed to the United Kingdom, which has rolled-out a similar adenovirus vaccine, made by AstraZeneca, that protects against severe disease, hospitalizations and deaths very well but is less effective against mild and moderate disease. Although the AstraZeneca vaccine is two doses, the UK has chosen to give the doses 12 weeks apart in order to vaccinate more people – a "one-dose-first" strategy that has been very effective, said Gandhi.
“Their hospitalizations and cases are plummeting faster just because they have more vaccine supply. Their outcomes have been phenomenal,” said Gandhi. “So when I look at that, I think, ‘Oh, I can quibble all I want about the clinical trial, but the real world is the real world.’”
What do we know about each vaccine’s ability to protect against the variants?
We know more about how the J&J vaccine protects against the coronavirus variants because the trials were conducted in South Africa and Brazil when the new variants had become prevalent. In South Africa, some 95 percent of circulating virus was the B.1.351 variant and in Brazil, 69 percent of the circulating virus was a P1/P2 variant at the time of the trial. Although the J&J vaccine appeared to be less effective against mild and moderate disease in these regions, it remained strongly protective against severe disease, hospitalizations and deaths.
“We cannot say definitively that the difference in efficacy in these regions is due to more variants, but it’s an assumption,” said Gandhi.
In the U.S., where the prevalence of variants was low, the overall efficacy of 72 percent likely was not affected by the variants, said Gandhi.
In contrast, the mRNA vaccine trials were not conducted in the presence of high levels of the variants, so less is known about how well they protect against the variants. Real-world data from Pfizer vaccinations suggest they are effective against the B.1.1.7 variant originally detected in the U.K.
What do we know about each vaccine’s ability to prevent asymptomatic infection?
We also have more information about asymptomatic infections from the J&J trials because they included asymptomatic PCR testing and antibody testing. (Participants were tested for antibodies to a part of SARS-CoV-2 that was not the spike protein since the vaccine would induce antibodies to the spike protein.) The vaccine was found to be 74 percent effective against asymptomatic infection, similar to its protection against symptomatic infections.
The mRNA vaccine trials did not look for asymptomatic infections, but Gandhi thinks all the vaccines likely offer parallel protection against symptomatic and asymptomatic infections. “They didn’t do this kind of testing, but based on the real-world data from vaccine roll-out, it seems likely your protection against asymptomatic infection will probably mirror your protection against symptomatic infection,” she said.
A study of U.K. health care workers, for example, found that the Pfizer vaccine reduced all infections, including asymptomatic, by 86 percent. A real-world study of the general population in Israel found that the Pfizer vaccine reduced asymptomatic infection by 94 percent. Another study, among pre-surgical patients across the Mayo Clinic system, showed that mRNA vaccines were 80 percent protective against asymptomatic infections.
Were there other differences between the vaccine trials?
Compared to the mRNA vaccine trials, the J&J vaccine trial included more participants who identified as Hispanic/Latino (45.1 percent) and Black or African American (17.2 percent). It also included more participants who were over 60 years old (34.6 percent) and more who had comorbidities (39.9 percent).
The more diverse participants not only help to better determine vaccine efficacy but also “enhances trust among the populations that look like the populations who are enrolled,” said Gandhi.
If you’re somewhat less protected against mild and moderate COVID-19 with the J&J vaccine, does that mean you are more likely to transmit the virus? Should your behavior depend on which vaccine you get?
No vaccine can prevent all COVID-19 infections. To minimize the chances of transmitting the virus even after you’ve been fully vaccinated, Gandhi suggests avoiding contact with unvaccinated people if you experience possible COVID-19 symptoms.
The good news is that vaccination seems to reduce viral load in the small percentage of people who still become infected. Preliminary data from Israel of the Pfizer vaccine suggest that if you catch COVID-19 after vaccination, you’re likely to harbor less virus, making you less likely to transmit.
“I think it’s going to end up being quite hard to transmit virus after you’ve been vaccinated, even if you are exposed, because of the low viral load in your nose,” said Gandhi. “Which is one of the reasons the CDC has said that vaccinated people do not need to quarantine after exposure if asymptomatic. That’s really based on that data."